Various Images Created With Blender
These are images generated for various purposes using Blender just before AI became ubiquitous. AI can do a good job in general but becomes difficult to use once some specific and scientifically accurate details are needed. We now focus only on the specific molecular interactions that AI can’t do… yet.
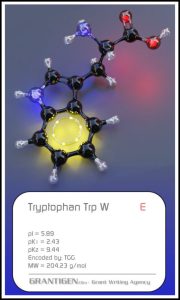
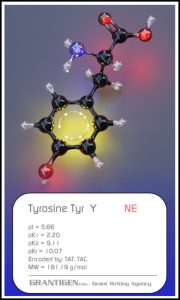
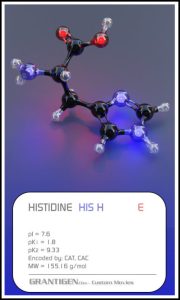
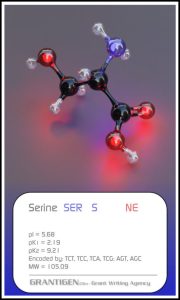
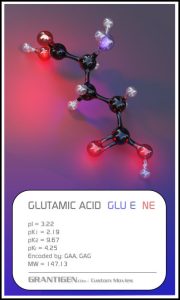
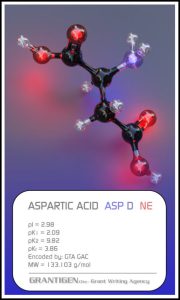
Amino Acids
Here are the 20 protein forming amino acids. Once they link together they are no longer 20 but one flesh.
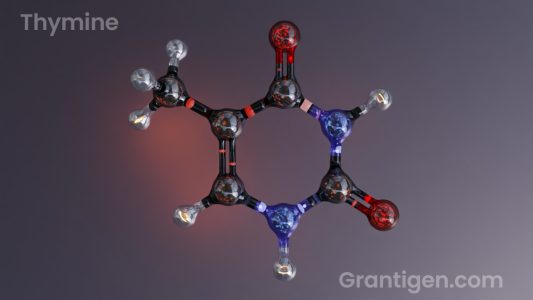
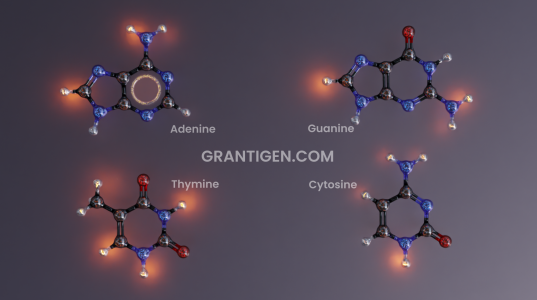
DNA Bases
There are only three letters in the DNA code, yet it is enough to write everything needed to sustain life.
Animated DNA Bases
These movies were made “by hand” using Blender. We decided to improve the images above with animation. Here is a tutorial that explains how to make the glowing orb in blender that we used to represent the atoms. The molecules are transparent in these videos. Here is a tutorial on how to make transparent glassy objects. From these videos on the animations it is obvious how much time and effort it takes to make animations in the “old fashioned way”.
Everything is much easier now, but unfortunately, achieving a high level of details and accuracy for some projects is difficult using AI only. This is because writing a prompt that will make AI generate a movie that one envisioned is a skill on its own plus every wrong move burns credits meaning that trial and error method is not free.
New AI models are getting better by the day. We shall see what the future holds. If AI can’t produce what you have envisioned – come to us. AI will not completely replace animators, but instead reduce the cost of movie production for everyone.